Have any questions?
get in touchTel : (86) 592-680-7188
E-mail : intecproducts@asintec.com
On May 29, 2024, InTec PRODUCTS announced that its independently developed and manufactured Malaria Pf and Malaria Pf/Pv RDTs have successfully passed the WHO Prequalification (PQ) certification. These products have now been officially included in the WHO's recommended procurement list for in vitro diagnostic products. This significant milestone marks InTec as the first Chinese company globally to obtain Malaria RDT WHO PQ certification. Furthermore, InTec currently holds the highest number of WHO PQ certifications among all Chinese companies, underscoring its leading position in the industry.
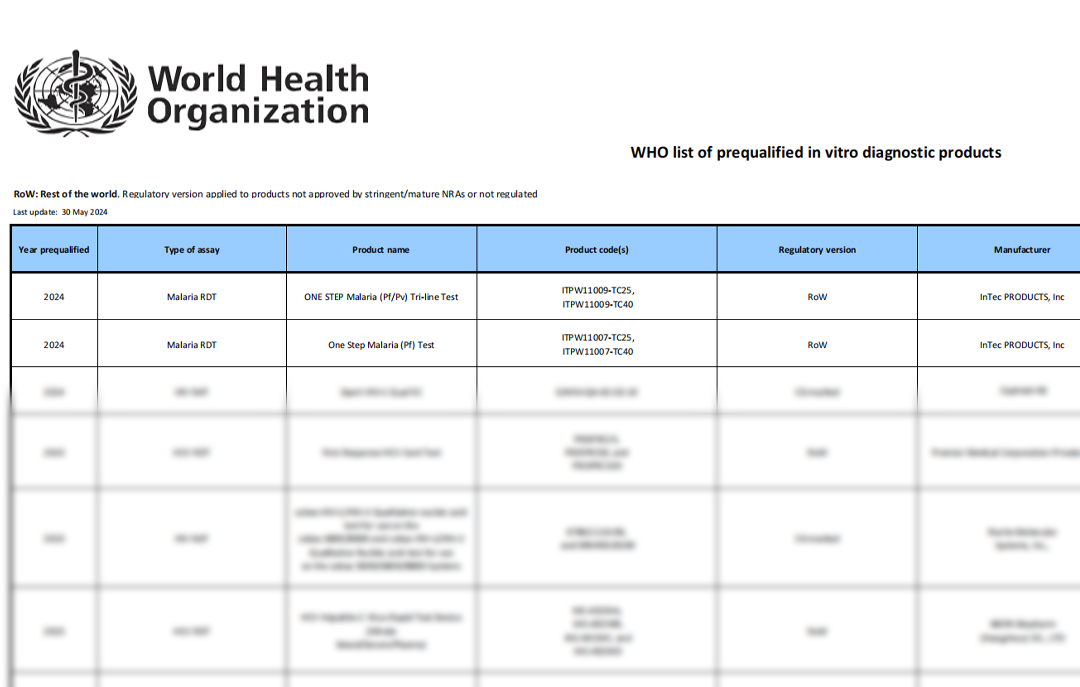

The WHO PQ process thoroughly assesses the
test's technical performance and manufacturing sites. Achieving
prequalification means the product meets international standards for quality,
safety, and performance, and it informs global funders and Ministries of Health
that the product is approved for procurement.

In
2019, InTec became the first Chinese company globally to obtain WHO PQ
certification for both HIV and HCV rapid diagnostic tests simultaneously. For
years, InTec has been providing high-quality and cost-effective diagnostic
products to international organizations such as UNICEF and USAID, earning an
excellent brand reputation.
Now, five years later, InTec's malaria RDTs have achieved two additional WHO PQ certifications. Following the success of its HIV and HCV products, these new certifications represent another significant milestone, further supporting and ensuring global public health efforts.
